EXTEND SURVIVAL AND DELAY DISEASE PROGRESSION FOR LONGER THAN ADT ALONE1
_tablets.jpg?width=480&height=95&format=jpg&quality=50)

SAVE OTHER TREATMENTS FOR LATER STAGES OF THE DISEASE1
ERLEADA® is the only novel antiandrogen approved in prostate cancer that provides PFS2 data from a broad population of patients with mHSPC, regardless of their disease status at treatment initiation‡1–4 – allowing you to plan for the future and keep other treatments for later stages of disease.1
- Hormonal therapy
- Chemotherapy
- Other approved therapy
INTERVENE EARLY. TREAT WITH ERLEADA® + ADT IN mHSPC6
ERLEADA + ADT:
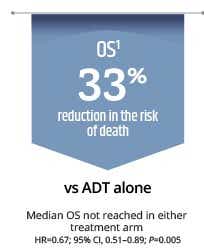
- Significantly extends overall survival vs ADT alone1
- Significantly delays rPFS vs ADT alone1
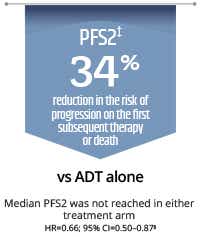
- Delays time to PSA progression and prolongs PFS2 vs ADT alone§1
- Provides an established safety profile that is comparable to ADT alone1
- Maintains HRQoL vs baseline1,6
- Provides easy to use, once-daily oral dosing7
ADT=androgen deprivation therapy; HRQoL=health related quality of life; mHSPC=metastatic hormone-sensitive prostate cancer; OS=overall survival; PFS2=second progression-free survival; PSA=prostate-specific antigen; rPFS=radiographic progression or death *OS and rPFS were dual primary endpoints of the TITAN study.1 **Radiographic progression-free survival: Time from randomisation to first imaging-based documentation of progressive disease or death, whichever occurred first.1 †TITAN is a robust, large-scale, double-blind, randomised, placebo-controlled international Phase III study that included a broad population of patients with mHSPC, regardless of their disease status at baseline, such as: high or low risk disease, high or low risk disease volume, Gleason score ≤7 or >7, previous docetaxel vs no previous docetaxel, de novo metastatic disease or relapsed metastatic disease after initial diagnosis or localised disease or, previous docetaxel or no previous docetaxel.1 ‡PFS2: Time from randomisation to the first occurrence of investigator-determined disease progression (PSA progression, progression on imaging, or clinical progression) while the patient was receiving first subsequent therapy for prostate cancer or death due to any cause, whichever occurred first.1§P values were not provided for time to PSA progression or PFS2.1,5
ERLEADA® INDICATION7
ERLEADA® is indicated in adult men for the treatment of non-metastatic castration-resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease. Medical castration with gonadotropin releasing hormone analogue (GnRHa) should be continued during ERLEADA® treatment in patients not surgically castrated.
References:
1. Chi KN, et al. N Engl J Med. 2019;81(1):13–24.
2. Armstrong AJ, et al. J Clin Oncol. 2019; ePub ahead of print. doi.org/10.1200/JCO.19.00799.
3. Fizazi K, et al. N Engl J Med. 2017;377:352–60.
4. Davis ID, et al. N Engl J Med. 2019; 381:121–31.
5. Chi KN, et al. N Engl J Med. 2019;81(1):13–24. Supplementary information.
6. Agarwal N, et al. Lancet Oncol. 2019; ePub ahead of print. doi.org/10.1016/S1470-2045(19)30620-5.
7. ERLEADA® (apalutamide) summary of product characteristics. Janssen-Cilag International NV, Beerse, Belgium, [MONTH] 2019.
CP-194717