SIGNIFICANT IMPROVEMENT IN 7 OF 8 QUALITY OF LIFE DOMAINS versus placebo



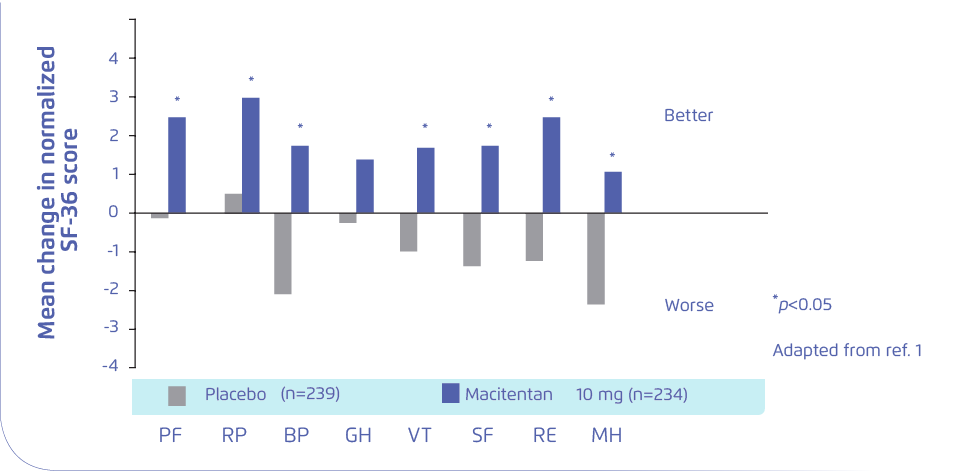
*SERAPHIN was a multicenter, double-blind, placebo-controlled, longterm, event-driven, phase III trial of macitentan in patients with PAH to evaluate the effect of macitentan on HRQoL in patients with PAH in the Study with an Endothelin Receptor Antagonist in Pulmonary Arterial Hypertension after 6 months, 12 months and at the end of treatment to Improve Clinical Outcome. 710 patients were included in this
analysis and received placebo, macitentan 3 mg, or macitentan 10 mg. (p<0.05) PF: Physical functioning; RP: Role physical; BP: Bodily pain; GH: General health; VT: Vitality;
SF: Social functioning; RE: Role-emotional; MH: Mental health
Significant increase in 6MWDa at 6 months compared to placebo
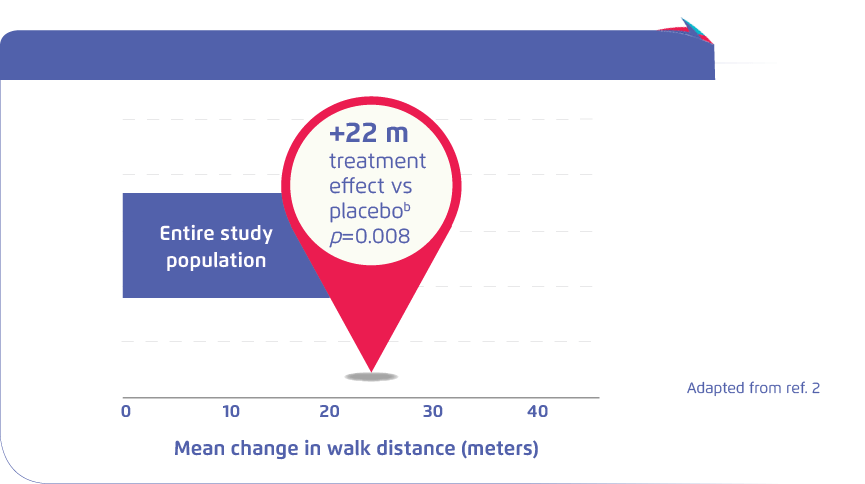
aThe change from baseline to month 6 in the 6-minute walk distance was evaluated as prespecified secondary end points.
bMean change versus placebo
6MWD: 6-minute walk distance
2. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. NEngl J Med. 2013; 369 (9): 809-18.
CP-250196
